On June 15, 2025, the 8th China Regulatory Science Conference convened in Beijing. Cao Gang, Director of the Western Medicine Department at the China Chamber of Commerce for Import and Export of Medicines and Health Products, was invited to deliver a keynote speech titled “Trends and Reflections on Domestic and International Pharmaceutical Supply Chain Development” at Forum 6: “Pharmaceutical Distribution and Regulatory Innovation.” The following is a transcript of his speech.

The pharmaceutical production and supply chain, serving as the core link connecting drug R&D, manufacturing, distribution, and consumption, is the “invisible lifeline” safeguarding public health. Its stability and efficiency not only impact the sustainable development of healthcare systems but have also become focal points amid global transformations—from capacity restructuring triggered by geopolitical conflicts to AI and blockchain-driven technological revolutions; from demand stratification spurred by aging populations to green transitions under the dual carbon goals. Amidst the current interplay of challenges and opportunities, the industry stands at a pivotal moment for breakthrough innovation.
Overview of Domestic and International Pharmaceutical Supply Chains
(I) Global Pharmaceutical Supply Chain Development Status and Trends
The global pharmaceutical market continues to expand, reaching approximately $1.57 trillion in 2023, $1.67 trillion in 2024, and projected to grow to $1.77 trillion by 2025, with a compound annual growth rate (CAGR) of 4.8%. The United States, China, the European Union, and Japan dominate the market, with the U.S. maintaining its position as the world’s largest pharmaceutical market and China ranking second. The rise of the CXO industry and new drug approvals serve as primary drivers.
Focus on the U.S. Market The U.S. is strengthening supply chain autonomy and promoting the return of manufacturing. While American innovative drugs are highly competitive, essential medicines—particularly active pharmaceutical ingredients (APIs)—remain heavily reliant on China and India. Data indicates that 80% of U.S. non-patent drug API production facilities are located overseas, with 45% of critical starting materials entirely dependent on China. In 2023, among overseas manufacturing sites regulated by the U.S. FDA, India hosted 585 facilities—a 16% increase over five years—while China hosted 484, marking a 25% rise over the same period.
Despite U.S. efforts since 2021 to promote localization through policies like the American Supply Chain Executive Order and the Bioactives Act, progress has been limited. In 2025, the Trump administration used tariffs to pressure pharmaceutical companies to return, prompting multiple multinational drugmakers to announce domestic investment plans. Concurrently, the U.S. Department of Defense classified drugs sourced from China as “extremely high risk,” accelerating efforts to “de-China-ize” the supply chain.
On May 5 this year, Trump signed the “Streamlining Regulation to Promote U.S. Critical Drug Manufacturing” executive order, directing the FDA to simplify reviews for domestic drug production while intensifying inspections of foreign manufacturing facilities.
From the European Market Perspective Europe is grappling with drug shortages and innovation outflow. As the birthplace of modern pharmaceuticals, Europe has seen innovation decline due to long-term price controls, with the number of globally approved innovative drugs dropping from 21 in 2015 to 11 in 2024. Facing cost pressures from China and India, some pharmaceutical companies are shifting to overseas markets, such as expanding their presence in China. In 2020, the EU released the European Pharmaceutical Development Strategy, aiming to address issues like insufficient manufacturers and fragile supply chains.
From the perspective of new global growth poles
Japan, the world’s third-largest pharmaceutical market with a 2023 market size of $109 billion, supports domestic production through investment subsidies and overseas supply chain diversification policies. This drives capacity shifts from specific countries to Southeast Asia, increasing supply sources.
India, the world’s largest generic drug producer accounting for 40% of the U.S. generic market, relies on China for 70% of its active pharmaceutical ingredients (APIs). Since 2020, the Indian government has implemented policies like the “Pharmaceutical Production-Linked Incentive Scheme” to encourage domestic drug manufacturing and reduce dependence on imported APIs.
South Korea has set a goal to become the world’s top biopharmaceutical manufacturing nation by 2030. In April 2025, it enacted the Synthetic Biology Promotion Act, the world’s first standalone legislation dedicated to advancing synthetic biology. By 2024, South Korea ranked fourth globally in the number of clinical trials conducted.
The Middle East and North Africa region is experiencing rapid growth in pharmaceutical consumption. Countries including Saudi Arabia and the United Arab Emirates are incentivizing local supply chain development through expedited approvals and preferential pricing.
(II) From the Perspective of China’s Pharmaceutical Supply Chain Development Status and Trends
As a key player in the global pharmaceutical industry chain, China accounts for 20.3% of the global pharmaceutical market, with generic drugs comprising 23.3% and innovative drugs 6.7%. This has formed a progressive overseas expansion pattern of “API → generic drugs → innovative drugs.”
Active Pharmaceutical Ingredients (APIs) Sector China’s API exports surged from $23.55 billion in 2015 to $42.98 billion in 2024, achieving a compound annual growth rate (CAGR) of 7.7%. With production capacity accounting for one-third of the global total, China stands as the world’s largest API producer and exporter. Primary export destinations include the EU, the United States, and India, with antibiotics, vitamins, and hormones constituting the bulk of exports.
Generic Drugs Sector The past decade has witnessed rapid growth in overseas expansion. Initiated by the 2015 generic drug consistency evaluation program, which elevated generic drug quality standards, Chinese pharmaceutical companies have secured over 700 ANDA approvals from the U.S. FDA by 2024.
Innovative Drugs Sector The sector has transitioned from “scale-driven” to “original innovation.” In 2024, 81% of IND applications for therapeutic biologics were innovative. Ten cell and gene therapies were approved, including six CAR-T therapies. License-out transactions reached 94 deals worth $51.9 billion in 2024, with 41 deals totaling $36.9 billion in Q1 2025—setting a new record for the period. Overseas expansion models shifted from “licensing clinical development + licensing commercialization” to “in-house clinical development + collaborative/in-house commercialization,” with traditional pharmaceutical companies increasingly prioritizing direct control over overseas markets.
Technological Trends and Developments
AI permeated R&D and production, while green manufacturing processes like cell therapy and microchannel continuous flow gained widespread adoption. Emerging markets such as Southeast Asia, Belt and Road regions, and the Middle East became key expansion targets.
Operational Performance of China’s Pharmaceutical Industry Imports and Exports
(I) Analysis of Pharmaceutical Foreign Trade Trends
In 2024, China’s total pharmaceutical trade reached $199.376 billion, comprising $91.41 billion in imports and $107.96 billion in exports, maintaining a trade surplus.
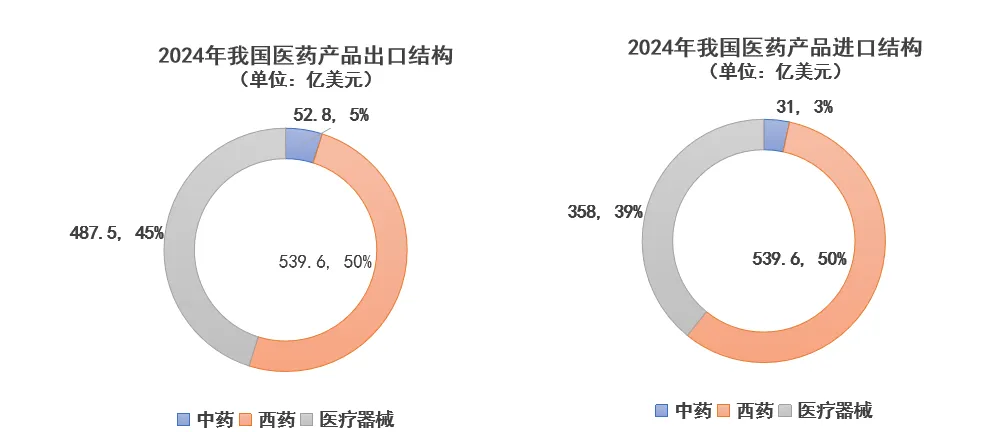
In the export structure, traditional Chinese medicine accounted for 5.28 billion yuan (5%), Western medicine for 53.96 billion yuan (50%), and medical devices for 48.75 billion yuan (45%); in the import structure, traditional Chinese medicine accounted for 3.1 billion yuan (3%), Western medicine for 52.5 billion yuan (57%), and medical devices for 35.8 billion yuan (40%). The import and export structure has remained stable for many years.
(II) Export Markets
Comparing pre- and post-pandemic data reveals minimal changes, with the top five markets remaining the European Union, the United States, ASEAN, India, and Japan. Specifically: – Exports to the United States reached $19.05 billion, up 11.7% year-on-year. – Exports to the EU totaled $24.29 billion, increasing by 6.7% year-on-year. – Exports to ASEAN amounted to $11.91 billion, growing by 5.7% year-on-year. – Exports to India were $8.353 billion, rising 1.2% year-on-year. – Exports to Japan stood at $5.46 billion, declining by 4.6% year-on-year.
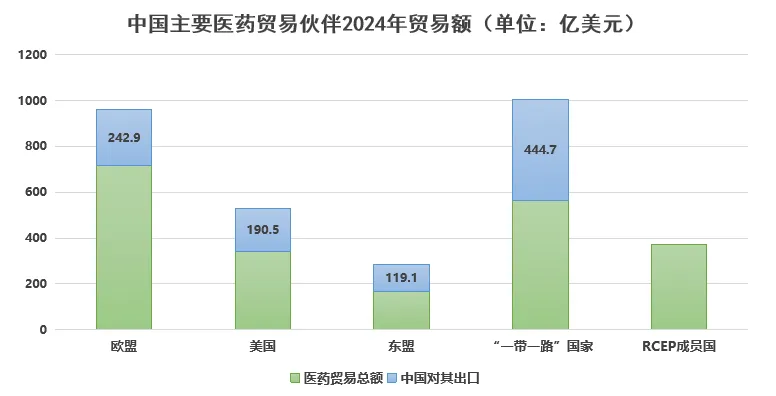
Additionally, exports to Belt and Road countries reached $44.47 billion, up 7.7% year-on-year, while exports to emerging markets such as Africa grew by 11.4% and those to Russia increased by 5.3%.
(Ⅲ) Industry Performance Trends Since 2025
From January to April 2025, China’s pharmaceutical product exports reached $35.85 billion, up 5.2% year-on-year, while imports totaled $28.3 billion, down 5.1% year-on-year. The structure of product imports and exports remained consistent with that of 2024.
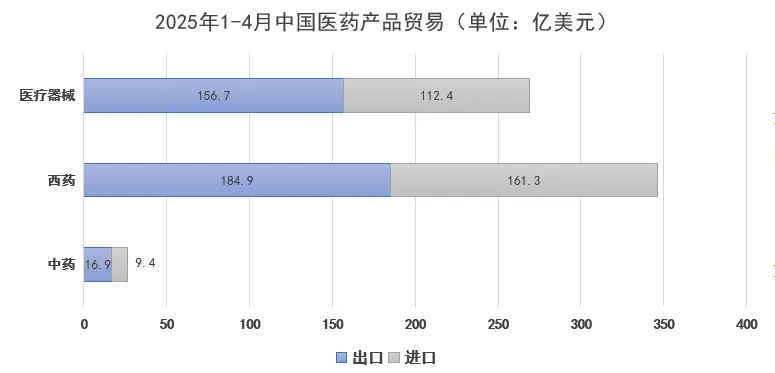
Among these, traditional Chinese medicine exports reached $1.69 billion, while imports totaled $940 million; Western medicine exports amounted to $18.49 billion, with imports at $16.13 billion; medical device exports stood at $15.67 billion, and imports at $11.24 billion.
Among export markets, the EU remained the largest market at $8.46 billion, up 6.4% year-on-year, followed by the United States at $5.96 billion and ASEAN at $3.9 billion. Exports to Belt and Road countries reached $14.78 billion, a 6.3% increase year-on-year, with significant growth observed in exports to Pakistan, Brazil, Egypt, and Russia.
Current Status of China-US Pharmaceutical Trade Under Tariff Measures
(I) Overview of US Tariff Impositions
Historical Development
2018 marked the early phase of the China-US trade war, with only select medical devices and pharmaceutical intermediates subject to 7.5%-25% tariffs. In 2021, the Biden administration maintained these tariffs, raising Section 301 tariffs on medical surgical gloves and syringes to 50% and 100% respectively starting January 2025, and further increasing the Section 301 tariff on medical surgical gloves to 100% starting January 2026.
From the perspective of the Trump administration’s current term: Starting February 2025, the U.S. first imposed a 20% tariff on all Chinese imports to the U.S. citing the “fentanyl issue” (initially 10% in February, raised to 20% in March).
Starting in April, reciprocal tariffs were imposed and escalated progressively. The U.S. gradually raised its reciprocal tariffs on China from 34% to 125%. Following the May Joint Statement of the U.S.-China Economic and Trade Talks in Geneva, 91% of reciprocal tariffs were eliminated, 24% reciprocal tariffs were suspended for 90 days, and the current phase implements 10% reciprocal tariffs. The U.S. reciprocal tariff exemption list covers the vast majority of pharmaceutical preparations and goods explicitly designated as active pharmaceutical ingredients (APIs), but medical devices and certain pharmaceutical intermediates remain exempted.
The U.S. “Section 301 tariffs,” “fentanyl tariffs,” and “reciprocal tariffs” are calculated cumulatively. Following the Geneva Joint Statement, applying the cumulative calculation rule to current tariff levels yields the following examples: syringes exported to the U.S. face a combined tariff rate of 130% after applying Section 301, fentanyl, and reciprocal tariffs. Amoxicillin API faces only the 20% fentanyl tariff rate. Pharmaceutical preparations containing norephedrine incur a combined 30% rate from the fentanyl and reciprocal tariffs. Medical devices like magnetic resonance imaging (MRI) equipment face a final rate of 55% after layering Section 301, fentanyl, and reciprocal tariffs.
Although U.S. reciprocal tariffs exempt most pharmaceutical preparations and goods explicitly classified as APIs, the U.S. is currently conducting a Section 232 investigation into the pharmaceutical industry, which may lead to further expansion of the tariff scope in the future.
(II) China-U.S. Pharmaceutical Trade Amid the Trade War
2024 Bilateral Trade Performance
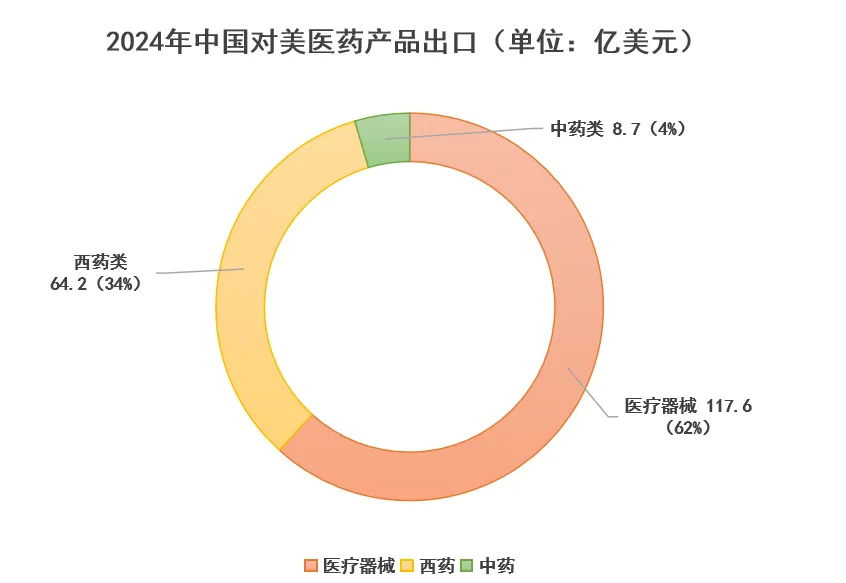
China’s pharmaceutical exports to the U.S. reached $19.05 billion, marking an 11.7% year-on-year increase and accounting for 17.6% of total exports. This comprised $11.76 billion in medical devices (61.7%), $6.42 billion in Western medicines (33.7%), and $870 million in traditional Chinese medicines (4.6%).
Exports of active pharmaceutical ingredients (APIs) to the U.S. reached $4.52 billion, up 12.1% year-on-year, primarily consisting of vitamins, peptide hormones, and amino acids. Imports of APIs from the U.S. totaled $840 million, increasing by 16.8% year-on-year, mainly comprising antibiotics and hormones. Pharmaceutical exports reached $1.9 billion, up 18.3% year-on-year, primarily consisting of immunological products and coenzyme Q10. Pharmaceutical imports totaled $5.5 billion, down 1.7% year-on-year, mainly comprising immunological products and vaccines.
Medical device exports to the U.S. amounted to $11.76 billion, increasing 9.4% year-on-year, with exports dominated by massage appliances and medical consumables. Imports reached $8.52 billion, primarily consisting of diagnostic reagents and high-end equipment.
Latest Trends Since 2025 From January to April this year, exports to the U.S. accounted for 16.7% of total exports, down 0.9 percentage points from 2024. Traditional Chinese medicine exports grew by 12.31%, Western medicine by 7.3%, and medical devices by 2.2%. March exports reached $1.62 billion, up 13.6% year-on-year, driven by volume growth—notably in medical dressings and vitamin C exports. April exports totaled $1.32 billion, down 11.4% year-on-year, reflecting pronounced fluctuations in both volume and price. Some products experienced declines in both volume and price, indicating the impact of tariffs on exports.
Global Pharmaceutical Production and Supply Chain Layout and Development Trends
(1) Coexistence of Globalization and Regionalization Accelerates Supply Chain Restructuring
Under globalization, knowledge-intensive nations (e.g., the U.S.) focus on innovation and R&D, while labor-intensive countries (e.g., China, India) handle production to enhance drug accessibility. However, trade protectionism and geopolitical factors are driving supply chains toward “localization, diversification, and decentralization.” Last year, the U.S. partnered with Japan, South Korea, India, and Europe to establish the Biopharmaceutical Alliance, committed to building a sustainable biopharmaceutical supply chain and repatriating manufacturing. This initiative will undoubtedly accelerate the reshaping of the global biopharmaceutical industry landscape.
(II) China’s Deep Integration into Global Industrial Chains
China hosts 12% of the world’s pharmaceutical R&D companies and accounts for 26.7% of global drug pipelines—second only to the United States. In cutting-edge fields like CAR-T therapy, China is running neck-and-neck with the U.S.
Regarding ASEAN and the Belt and Road Initiative: Driven by the RCEP, China’s pharmaceutical exports to ASEAN reached $11.9 billion in 2024, with a compound annual growth rate of 9.9%.
Regarding China-India competition and cooperation: In 2024, China exported $6.13 billion worth of active pharmaceutical ingredients (APIs) to India while importing $740 million. The two nations exhibit strong complementarity in generic drugs and APIs, maintaining both competitive and cooperative relationships.
Regarding China-EU cooperation: Numerous multinational pharmaceutical companies are engaging in joint innovative drug development with China, demonstrating highly complementary industrial chains between China and Europe.
Regarding China-US cooperation: The license-out model has become the core pathway for China’s innovative drugs to go global. In 2024, China-US cooperation transactions accounted for the highest proportion, with both sides deeply integrated in R&D and commercialization.
Corporate Response Strategies
(I) Diversified Market Expansion
Reduce reliance on any single market while deepening engagement in emerging markets to capture growth opportunities. Facing U.S. tariffs and supply chain risks, companies are actively accelerating expansion into emerging markets such as Southeast Asia, the Middle East, North Africa, and Latin America. They are building regional production and sales networks through technology transfer and joint R&D. Leveraging tariff concessions under the Belt and Road Initiative and expedited approval policies in the Middle East helps shorten product launch cycles.
(2) Production Base Relocation and Localized Manufacturing
To mitigate the impact of U.S. tariff hikes on multinational supply chains, companies with existing U.S. production facilities are evaluating the feasibility of shifting export production domestically. This “local production, local supply” approach enhances supply chain resilience.
(III) Flexible Overseas Expansion Models for Win-Win Collaboration
License-out Model: Leveraging existing platforms for rapid capital recovery, suitable for startups but requires guarding against “product return” risks.
NewCo Model: Collaborative overseas ventures testing cross-cultural management capabilities, demanding reliable partners.
In-house Team Model: Building independent capabilities for strategic control, ideal for industry leaders with R&D capacity, capital, and global vision.
Reflections and Recommendations
(I) Shifting the Industrial Chain Toward Higher Value-Added Products
China’s pharmaceutical exports have long relied on active pharmaceutical ingredients (APIs) and low-value-added products. In 2024, Western pharmaceutical preparations and biochemical drugs contributed 20% to the growth of pharmaceutical exports, exceeding their 10% share in export value, indicating structural optimization. Human vaccine exports surged 62.1% year-on-year in 2025 as domestic enterprises increasingly penetrated international markets. The industry chain is transitioning from “raw material-driven” to “innovation-driven,” though exports of APIs still account for 80% of total exports. This contrasts with the domestic industrial structure where APIs constitute only 15%, highlighting the need for further coordination.
(II) Overseas Expansion Models for Innovative Drugs and Industrial Chain Security
The license-out model gained momentum, with multiple major deals closing in 2025. This model operates under the principle of “R&D in China, production overseas, and global markets.” However, it carries supply chain security risks: a high proportion of early-stage project licensing may weaken domestic R&D capabilities in later stages; regulatory perspectives on licensing vary across departments, lacking a unified monitoring mechanism. A monitoring system for innovative project licensing must be established to balance supply chain security and innovation development.
(III) Emerging Market Expansion and Transshipment Trade Risks
Emerging markets like the Middle East, Africa, and Southeast Asia have become crucial directions for countering tariff impacts. By 2025, some enterprises will maintain operations by shifting markets—for instance, Europe absorbing U.S. market share. However, re-export trade carries risks: Vietnam is strengthening origin verification, and certain nations may face additional tariffs under U.S. pressure. Vigilance is needed against compliance risks in “detour” exports. Enterprises must be guided to expand markets compliantly and diversify supply chain layouts.
Conclusion
The evolution of pharmaceutical production and supply chains has never been linear; it has always been a leap across discontinuities. While concerns over volume-based procurement pricing pressures persist, the global expansion of innovative drugs is opening new avenues. As Europe and the U.S. erect supply chain barriers, the Belt and Road Initiative is paving new pathways. When traditional models hit their limits, digital and intelligent transformation is reshaping foundational logic.
At this historical inflection point, we must harness “resilience” to weather geopolitical storms; employ ‘innovation’ to breach technological barriers; and foster “symbiosis” to build an inclusive, shared global ecosystem.
The ultimate value of the pharmaceutical supply chain lies not in cold efficiency metrics, but in the warmth of safeguarding lives. Let us join hands to fortify an even more robust “Great Wall of Supply Chains,” ensuring the health and well-being of humanity!



